Abstract
Introduction: Pregnancy requires, and is an important motivator of tyrosine kinase inhibitor (TKI) cessation in patients with chronic myeloid leukemia (CML). While conventional treatment free remission (TFR) attempts may allow observation of limited rise in BCR-ABL prior to expected TKI re-exposure, TKI cessation in pregnancy affords longer observation of BCR-ABL kinetics without automatic TKI re-exposure. Mathematical models and clinical observation of BCR-ABL kinetic rise during TKI discontinuation or planned cessation estimate of 'doubling time' (DT) of roughly 9 days (Branford et al., Blood 2012). In order to explore the impact of pregnancy, we studied BCR-ABL kinetics and response stability during and after pregnancy.
Methods: We collected cases of successful pregnancies (conception->childbirth) at 4 CML referral centers including the following conditions: 1/conception occurring while on TKI therapy; 2/TKI therapy stopped for the purpose of conception; and 3/ pregnancy during TKI cessation within a TFR clinical trial. Cases with early spontaneous/elective abortion, treated with interferon during pregnancy or with less than 2 BCR-ABL transcripts recorded during pregnancy were excluded. Doubling time (DT) was calculated using the following formula: DT = ln2/k, where k = (ln(b)-ln(a))/d, where (a) and (b) is the value before the rise and at the rise, and (d) is days.
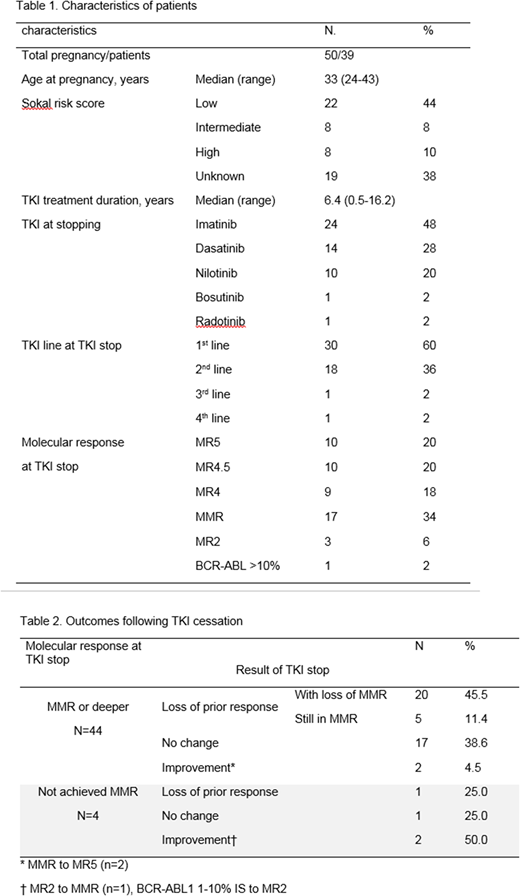
Results: In total 50 pregnancies in 39 patients were analyzed; 10 patients had >1 pregnancy. Four pregnancies were in the context of TFR study (2 enrolled at conception, 1 patient 28mo in TFR, 1 patient 5mo in TFR). The majority of cases were on first-line treatment at TKI cessation and median duration of TKI therapy was 6.4 years (range 0.5-16.2); 58% were in deeper molecular response (MR4 or deeper) and 34% in major molecular response (MMR) at TKI cessation. Patient characteristics are summarized in Table 1.
Median time off TKI was 10.1 months (range 5.4-71.5). Of 44 pregnancy cases within MMR or deeper at TKI cessation, 54.5% maintained MMR or greater; 60.7% of those in MR4 or deeper and 43.7% for those in MMR, respectively. Several cases were associated with decline in BCR-ABL off TKI: 2 cases of improvement from MMR to deep MR (MR5), and among 4 cases not in MMR at TKI cessation, 1 achieved MMR during pregnancy (Table 2).
BCR-ABL rise in 2 or more consecutive measurements, and at least one measurement of rise defined as more than 2-fold increase, was observed in 24 patients. The median BCR-ABL doubling time among 54 such instances in these 24 patients was 18.3 days (range 1.8-306.8). Of 20 cases that lost MMR during pregnancy, 17 met these criterions for BCR-ABL rise; the median doubling time among 40 such instances in these cases was 14.7 days (1.8-306.8).
Postpartum (n=48), 34 cases have been retreated to date; 14 others remain off therapy with ongoing deep MR (MR5) in 6 cases, MMR in 6 and BCR-ABL <1 (MR2) in 2. Of the 34 cases of retreatment, 5 were not evaluable due to limited time back on TKI (n=3), immediate re-cessation of TKI due to second pregnancy (n=1) or loss of follow-up (n=1). Of the 29 evaluable cases, to date 22 achieved deep MR (MR4 or greater) and 7 achieved MMR. Three patients deemed to not respond adequately after prior TKI resumption switched TKI and achieved MMR or deeper.
Conclusions: BCR-ABL doubling time during TKI cessation for pregnancy was slower compared with that of non-pregnant patients with TKI cessation (TFR) or interruption historically. This retrospective analysis of pregnancy-associated TKI cessation demonstrates overall favorable response stability, with observation of high rates of MMR retention, slowing of BCR-ABL kinetic increase after initial rise, and rare cases of deepening remission over time off TKI. Overall kinetics of BCR-ABL appears variable during pregnancy associated TKI cessation. Given the prominence of pregnancy and family planning as a consideration for TFR attempt and these data, further study of the impact of pregnancy on CML biology, immune function, and relapse risk is warranted.
Kim:BMS: Research Funding; Ilyang: Research Funding; Pfizer: Research Funding; Novartis: Research Funding. Abruzzese:Ariad: Consultancy; Novartis: Research Funding; BMS: Consultancy; Pfizer: Consultancy. Apperley:Incyte: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Mauro:Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Takeda: Consultancy; Bristol-Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal